
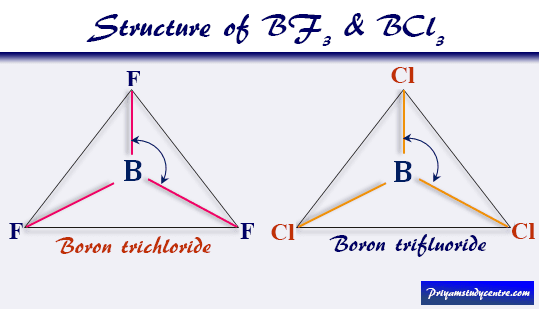
Although VSEPR theory predicts the distribution of the electrons, we have to take in consideration of the actual determinant of the molecular shape. Thus, the molecule's shape reflects its equilibrium state in which it has the lowest possible energy in the system. The electrons and the nuclei settle into positions that minimize repulsion and maximize attraction. The shape of a molecule is determined by the location of the nuclei and its electrons. Using the VSEPR theory, the electron bond pairs and lone pairs on the center atom will help us predict the shape of a molecule. An electron group can be an electron pair, a lone pair, a single unpaired electron, a double bond or a triple bond on the center atom. VSEPR focuses not only on electron pairs, but it also focus on electron groups as a whole. Thus, electron pairs will spread themselves as far from each other as possible to minimize repulsion. The valence-shell electron-pair repulsion (VSEPR) theory states that electron pairs repel each other whether or not they are in bond pairs or in lone pairs.
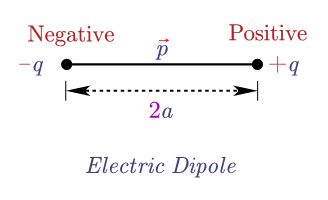

Now that we have a background in the Lewis electron dot structure we can use it to locate the the valence electrons of the center atom. Valence-Shell Electron-Pair Repulsion Theory


 0 kommentar(er)
0 kommentar(er)
